Abstract
Reduced-intensity conditioning (RIC) regimens for allogeneic blood and marrow transplant (alloBMT) offer lower treatment-related mortality than myeloablative conditioning. Fludarabine is a key agent for the majority of RIC and nonmyeloablative regimens, including in combination with cyclophosphamide, total body irradiation (TBI), and post-transplant cyclophosphamide (PTCy). Critical supply chain shortages of fludarabine highlight the need for substitute regimens. Herein we report the use of a novel RIC approach utilizing pentostatin as an alternative purine antimetabolite in adults receiving alloBMT for hematologic malignancies.
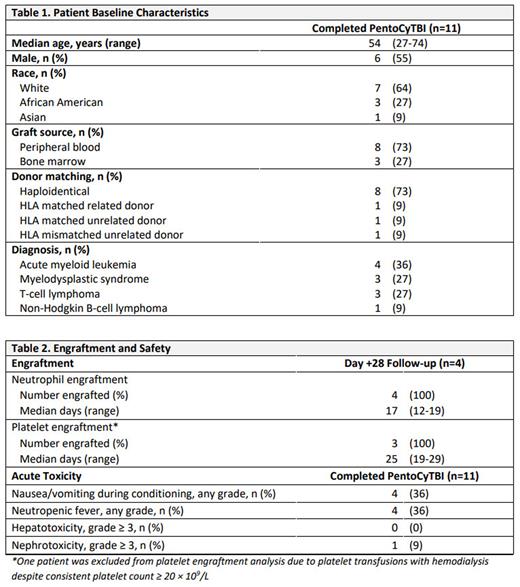
The RIC conditioning regimen included pentostatin 4 mg/m2 IV and cyclophosphamide 14.5 mg/kg IV on days -5 and -4 and TBI 400 cGy on day -1 (PentoCyTBI). Pentostatin was reduced by 50% for creatinine clearance < 50 mL/min. TBI 400 cGy was chosen over 200 cGy to maximize immunosuppression and engraftment. Graft-versus-host disease prophylaxis consisted of cyclophosphamide 50 mg/kg IV on days +3 and +4, mycophenolate mofetil beginning day +5 through day +35 and either tacrolimus or sirolimus beginning day +5 through day +60 to +180. Patients who were at least 28 days post-alloBMT were included in the engraftment analysis, while all patients who completed conditioning were included in the toxicity analysis. Neutrophil engraftment was defined as an ANC ≥ 0.5 × 109/L measured for three consecutive days and platelet engraftment was defined as a platelet count ≥ 20 × 109/L for three consecutive days without platelet transfusion in the preceding seven days.
Between 06/2022 and 07/2022, 11 patients received PentoCyTBI at The Johns Hopkins Hospital. The median follow-up was 16 days for all patients (range 8-36). Patient characteristics are summarized in Table 1. The median age was 54 years (range 27-74), 55% were male, and 36% were ethnic minorities. The most common indication for transplant was acute myeloid leukemia, with 73% of patients receiving peripheral blood as the stem cell source, and 73% from haploidentical donors. Of 4 patients with Day +28 follow-up, all had neutrophil engraftment at median 17 days (range 12-19) and 3 had platelet engraftment at median 25 days (range 19-29) (Table 2). One patient was excluded from the platelet engraftment analysis as platelet transfusions were continued while on hemodialysis despite achieving a stable platelet count ≥ 20 × 109/L. Nausea and vomiting during conditioning and neutropenic fever were the most common acute toxicities occurring in 4 patients (36%) each, though 3 cases of neutropenic fever were attributed to cytokine release syndrome. There were no reports of grade ≥ 3 hepatotoxicity. One patient experienced nephrotoxicity beginning Day +8, escalating to grade 4 requiring hemodialysis; conditioning included pentostatin dose reduction for baseline renal impairment and it is uncertain whether pentostatin contributed.
Options for alloBMT RIC without fludarabine are extremely limited. Early results using pentostatin as an alternate purine analog are encouraging, with full engraftment to date and similar timing to fludarabine-based regimens. Further patient volume and follow-up will be collected to assess long-term efficacy and safety outcomes.
Disclosures
Porter:Medexus Pharmaceuticals: Consultancy, Honoraria. Luznik:WindMil Therapeutics: Patents & Royalties; Genentech: Other: Clinical Trial Support, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rubius Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Talaris Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Ziggas:Seagen: Consultancy, Honoraria. DeZern:GERON: Other: DSMB; CTI BioPharma: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Syntrix Pharmaceuticals: Research Funding; Novartis: Consultancy, Honoraria. Fuchs:Iyuda Therapeutics, LLC: Consultancy, Current Employment, Other: Co-Founder.
OffLabel Disclosure:
Pentostatin is only FDA approved for hairy cell leukemia. This abstract presents data on pentostatin as a novel reduced-intensity conditioning regimen for allogeneic blood and marrow transplantation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal